Dr. Hauschka MED
Für besondere Bedürfnisse.
Dr. Hauschka MED steht für medizinische Pflegeprodukte aus der Natur. Die Produkte für sehr trockene oder neurodermitische Haut, Zähne und kribbelnde Lippen haben wir zusammen mit Ärzten und Forschungsinstituten entwickelt.
 Gesichtscreme Mittagsblume
Gesichtscreme Mittagsblume - tägliche Basispflege
- bei sehr trockener, empfindlicher Haut, auch bei Neurodermitis
- reduziert Spannungsgefühle und beugt Juckreiz vor
- auch für Babys geeignet
 Pflegelotion Mittagsblume
Pflegelotion Mittagsblume - tägliche Basispflege
- bei sehr trockener, spannender, empfindlicher Haut, auch bei Neurodermitis
- mildert Trockenheit und beugt Juckreiz vor
- auch für Babys geeignet
 Handcreme Mittagsblume
Handcreme Mittagsblume - schützende Basispflege
- bei sehr trockener, beanspruchter Haut, auch bei Neurodermitis
- mildert Hauttrockenheit spürbar
- auch für Kleinkinder geeignet
 Intensivcreme Mittagsblume
Intensivcreme Mittagsblume - reichhaltige Intensivpflege
- bei sehr trockener, schuppiger, juckender Haut, auch bei Neurodermitis
- reduziert Juckreiz und Hauttrockenheit
- auch für Babys geeignet
 Akutcreme Potentilla
Akutcreme Potentilla - beruhigende Akutpflege
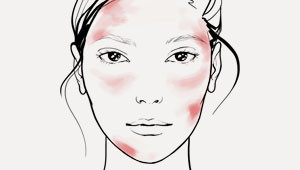
- bei gereizter, geröteter, juckender Haut, auch bei Neurodermitis
- reduziert Juckreiz und Rötungen schnell
- auch für Kleinkinder geeignet
 Shampoo Mittagsblume
Shampoo Mittagsblume - milde tägliche Reinigung
- bei trockener, empfindlicher Kopfhaut, auch bei Neurodermitis
- reinigt mild und pflegt
- sehr gut verträglich
 Cremeshampoo Lavaerde
Cremeshampoo Lavaerde - sanfte Reinigung und intensive Pflege
- bei sehr trockener, juckender, schuppiger Kopfhaut, auch bei Neurodermitis
- reinigt besonders schonend
- beruhigt Juckreiz schnell
Probiergrößenset
Teste die medizinische Systempflege von Dr. Hauschka MED.

 Kopfhautmaske Kürbiskern
Kopfhautmaske Kürbiskern - intensive Pflege und Beruhigung
- bei sehr trockener, juckender, schuppiger Kopfhaut, auch bei Neurodermitis
- mildert Spannungsgefühle und Trockenheit
- beruhigt die Kopfhaut nachhaltig
 Akut Lippenpflege
Akut Lippenpflege - bei Neigung zu Lippenbläschen
- kühlt und regeneriert
- wirkt beruhigend
- pflegt und beugt vor
 Minze Zahncreme forte
Minze Zahncreme forte - entfernt gründlich Zahnbelag
- wirkt entzündungshemmend
- für eine stabile Mundflora
- für einen frischen Atem
 Sole Zahncreme sensitiv
Sole Zahncreme sensitiv - für schmerzempfindliche Zähne
- schonende Reinigung
- beugt Zahnfleischbluten vor
- Homöopathie geeignet
 Salbei Mundspülung
Salbei Mundspülung - unterstützt die Zahnreinigung
- beugt Entzündungen vor
- stärkt und strafft das Zahnfleisch
- für einen frischen Atem
Das alles kann Dr. Hauschka MED.
Durch ihren schützenden und ausgleichenden Effekt leisten die Produkte von Dr. Hauschka MED mehr als eine normale Pflege. Sie arbeiten vorbeugend und stabilisieren zugleich. Dr. Hauschka MED umfasst Produkte für sehr trockene oder neurodermitische Haut, für Lippen, die zu Bläschen neigen, für den Schutz der Zähne und eine gesunde Mundflora. Das Herz unserer medizinischen Pflege sind Heilpflanzen, natürliche Öle und Wachse wie z.B. Mittagsblume, Avocadoöl oder Mangobutter.
Medizinische Pflege auf dem neuesten Forschungsstand.
Alle Produkte von Dr. Hauschka MED werden nach neuesten wissenschaftlichen Erkenntnissen in Zusammenarbeit mit Ärzten, Kosmetikerinnen, Test- und Forschungsinstituten entwickelt. Wissenschaftliche Studien belegen ihre Wirksamkeit. Die kompetente Basis dafür bildet die mehr als 80-jährige Erfahrung der WALA Heilmittel GmbH. Unter ihrem Dach werden sowohl die WALA Arzneimittel als auch Dr. Hauschka Kosmetik und Dr. Hauschka MED hergestellt.